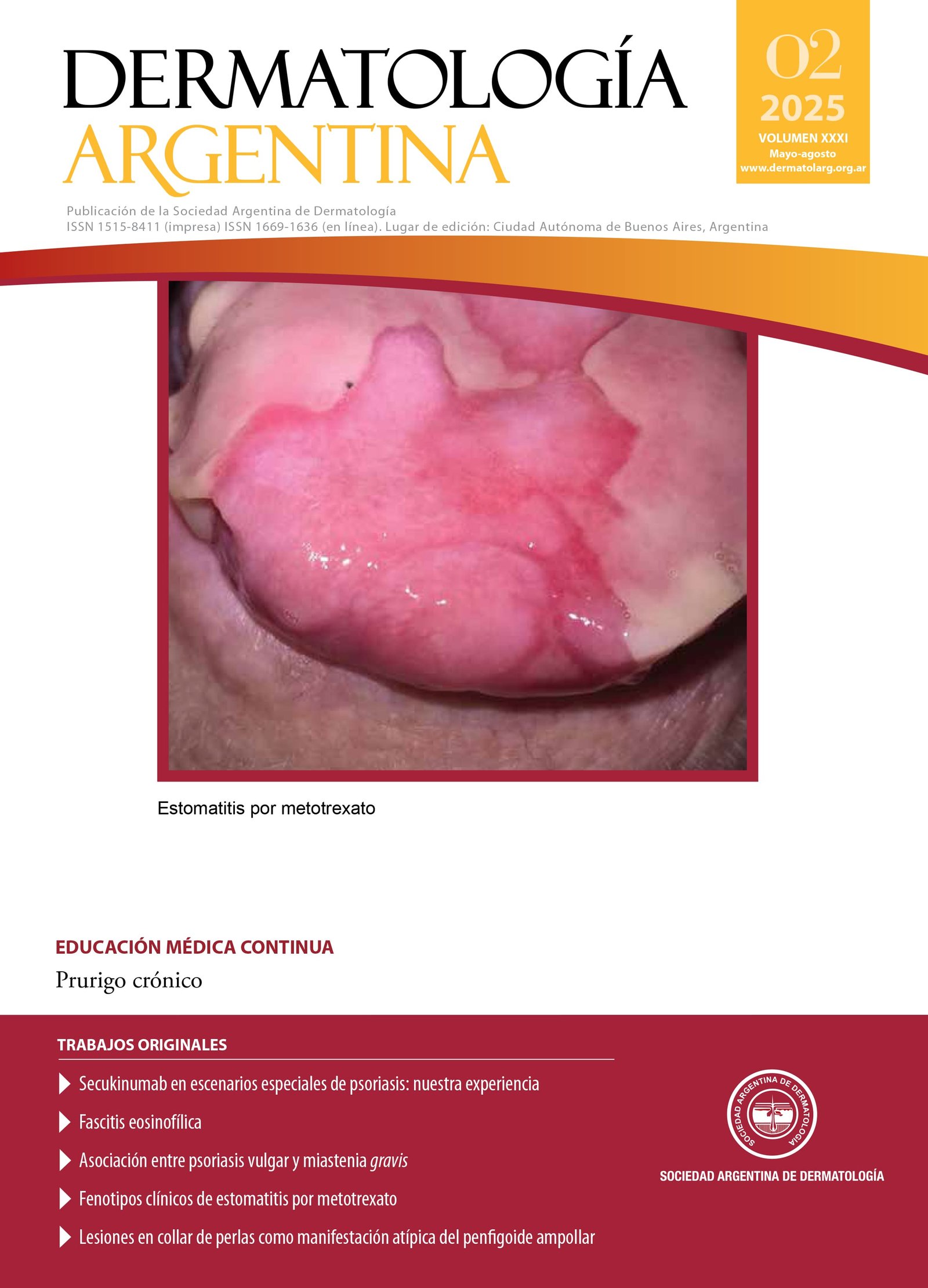
Clinical phenotypes of methotrexate-induced stomatitis
DOI:
https://doi.org/10.47196/da.v31i2.2870Keywords:
methotrexate, stomatitis, mucositis, aphthoid lesions, oral lichenoid reactionAbstract
Methotrexate (MTX) is an antimetabolite widely used to treat immune-mediated diseases such as rheumatoid arthritis and psoriasis. Its adverse effects include the development of mucocutaneous erosions and ulcers, which are considered early signs of systemic toxicity. The risk of these lesions is associated with factors such as overdose, renal insufficiency, and altered bioavailability. Oral manifestations, more common than cutaneous ones, include three clinical phenotypes: diffuse mucositis, irregular aphthoid lesions, and lichenoid reaction. This article describes two clinical cases with distinct oral toxicity phenotypes induced by MTX, highlighting the importance of early diagnosis and appropriate management. Furthermore, the need for future research to establish the correlation between clinical phenotypes of stomatitis and the risk of systemic involvement is emphasized.
References
I. Maiberger M, Nunley J, Wolverton S, et ál. Other systemic drugs. En: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Philadelphia: Elsevier; 2024:2323-2325.
II. Ferrari B, Kogan N, Veira RC, Luna PC, et ál. Manifestaciones mucocutáneas de intoxicación por metotrexato. Medicina (Buenos Aires). 2018;78:50-53.
III. Sherbini AA, Gwinnutt JM, Hyrich KL, Verstappen SMM, et ál. Rates and predictors of methotrexate-related adverse events in patients with early rheumatoid arthritis: results from a nationwide UK study. Rheumatol (Oxford). 2022;61:3930-3938.
IV. Primka EJ, Camisa C. Methotrexate-induced toxic epidermal necrolysis in a patient with psoriasis. J Am Acad Dermatol. 1997;36(5 Pt 2):815-818.
V. Borda LJ, Ross A, Villada G, Milikowski C. Acute mucocutaneous methotrexate toxicity with marked tissue eosinophilia. BMJ Case Rep. 2018;2018:bcr2017221489.
VI. Chen TJ, Chung WH, Chen CB, Hui RC, et ál. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2.
VII. Morgado-Carrasco D, Riquelme-Mc Loughlin C, Fustà-Novell X, Giavedoni P. Methotrexate-induced mucositis as a sign of bone marrow toxicity: a retrospective study of clinical and epidemiological characteristics. Actas Dermosifiliogr. (Engl Ed) 2020;111: 436-439.
VIII. Hamid M, Lashari B, Ahsan I, Micaily I, et ál. A deadly prescription: combination of methotrexate and trimethoprim-sulfamethoxazole. J Community Hosp Intern Med Perspect. 2018;8:149-151.
IX. Lee JS, Oh JS, Kim YG, Lee CK, et ál. Methotrexate-related toxicity in patients with rheumatoid arthritis and renal dysfunction. Rheumatol Int. 2020;40:765-770.
X. Schnabel A, Reinhold-Keller E, Willmann V, Gross WL. Tolerability of methotrexate starting with 15 or 25 mg/week for rheumatoid arthritis. Rheumatol Int. 1994;14:33-38.
XI. Rampon G, Henkin C, Jorge VM, Almeida HL, et ál. Methotrexate-induced mucositis with extra-mucosal involvement after accidental overdose. An Bras Dermatol. 2018;93:155-156.
XII. Magdy E, Ali S. Stratification of methotrexate-induced oral ulcers in rheumatoid arthritis patients. Spec Care Dentist. 2021;41:367-371.
XIII. Troeltzsch M, von Blohn G, Kriegelstein S, Woodlock T, et ál. Oral mucositis in patients receiving low-dose methotrexate therapy for rheumatoid arthritis: report of 2 cases and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e28-33.
XIV. da Silva Ferreira AR, van der Aa SAJ, Wehkamp T, Wardill HR, et ál. Development of a self-limiting model of methotrexate-induced mucositis reinforces butyrate as a potential therapy. Sci Rep. 2021;11:22911.
XV. Al-Jamaei AAH, Epstein JB, de Visscher JGAM, Spielberger RT, et ál. Comparing the risk of severe oral mucositis associated with methotrexate as graft-versus host-disease prophylaxis to other immunosuppressive prophylactic agents in hematopoietic cell transplantation: a systematic review and meta-analysis. Support Care Cancer 2024;32:519.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 on behalf of the authors. Reproduction rights: Argentine Society of Dermatology

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
El/los autor/es tranfieren todos los derechos de autor del manuscrito arriba mencionado a Dermatología Argentina en el caso de que el trabajo sea publicado. El/los autor/es declaran que el artículo es original, que no infringe ningún derecho de propiedad intelectual u otros derechos de terceros, que no se encuentra bajo consideración de otra revista y que no ha sido previamente publicado.
Le solicitamos haga click aquí para imprimir, firmar y enviar por correo postal la transferencia de los derechos de autor