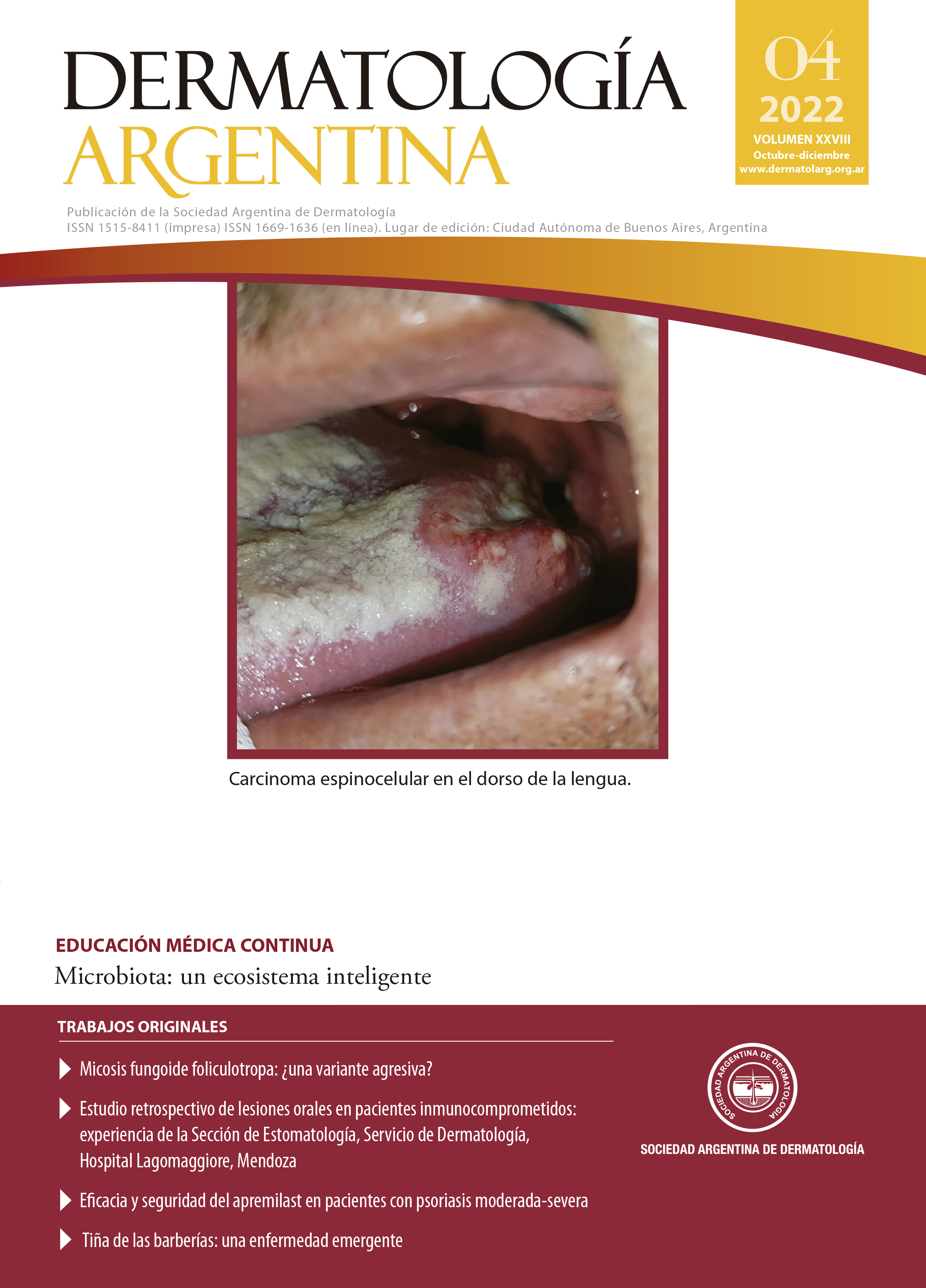
Efficacy and safety of apremilast in patients with moderate-severe psoriasis
DOI:
https://doi.org/10.47196/da.v28i4.2338Keywords:
psoriasis, apremilastAbstract
Background: apremilast, an oral inhibitor of phosphodiesterase 4, has been approved in our country to treat moderate to severe plaque psoriasis and psoriasis arthritis since 2017. Data on the efficacy and safety of apremilast in clinical practice are limited.
Objective: Review the efficacy considering PASI-75 response rate at week 16 and security in real-world clinical practice of apremilast at a third-level hospital in Argentina.
Design: all patients treated in the Hospital Italiano de Buenos Aires psoriasis department, who were prescribed apremilast between June 1 2019, and June 30 2022, were included.
Materials and methods: data were obtained from the electronic medical record and incorporated into a de-identified database designed for this work, with access restricted only to researchers.
Results: nineteen patients with plaque psoriasis were included. Six had mild disease and 12 had moderate-severe disease. 5/18 patients achieved the primary outcome (PASI 75 response). Treatment had to be discontinued on 11/19 due to adverse effects or treatment failure.
Conclusions: in our study, the efficacy of apremilast in real life was lower than reported in the pivotal studies.
References
I. Christophers E. Psoriasis-epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26: 314-320.
II. Chouela E, Amaya M, Londoño A, et ál. Psoriasis in Latin America. Dermatol Online J. 2016;22(9):13030/qt4wn3m8xt. [Consultado junio 2022].
III. Strober B, Ryan C, van de Kerkhof P, van der Walt J. et ál. International Psoriasis Council Board Members and Councilors. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council Board Members and Councilors. J Am Acad Dermatol. 2020;82:117-122.
IV. ANMAT. Principal. Disponible en: http://www.anmat.gob.ar/boletin_anmat/octubre_2017/Dispo_10552-17 [Consultado agosto 2022].
V. Trujillo A, Flamini M, Díaz P, García S, et ál. Reacción acneiforme como efecto adverso del apremilast. Dermatol Argent. 2021;25:28-30.
VI. Papp K, Reich K, Leonardi CL, Kircik L, et ál. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
VII. Paul C, Cather J, Gooderham M, Poulin Y, et ál. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
VIII. Del Alcázar E, Suárez-Pérez J.A, Armesto S, Rivera R, et ál. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicentre study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol. 2020;34:2821-2829.
IX. Ghislain PD, Lambert J, Hoai XLL Hillary T, et ál. Real-Life Effectiveness of Apremilast for the Treatment of Psoriasis in Belgium: Results From the Observational OTELO Study. Adv Ther. 2022;39:1068-1080.
X. Ioannides D, Antonakopoulos N, Chasapi V, Oikonomou C, et ál. A real-world, non-interventional, prospective study of the effectiveness and safety of apremilast in bio-naïve adults with moderate plaque psoriasis treated in the routine care in Greece - the “APRAISAL” study. J Eur Acad Dermatol Venereol. 2022;36(11):2055-2063.
XI. Nuevas Guías Argentinas de tratamiento sistémico de la psoriasis moderada a severa 2020. Disponible en: http://www.soarpso.org/recursos/archivos/AG_psoriasis_2020_espanol. [Consultado septiembre 2022].
XII. Consenso Nacional de Psoriasis. Guía de tratamiento, actualización 2022. Sociedad Argentina de Dermatología. Disponible en: https://sad.org.ar/wp-content/uploads/2022/08/CONSENSO-NACIONAL-PSORIASIS-web.pdf. [Consultado octubre 2022].
XIII. Nast A, Smith C, Spuls PI, Avila Valle G, et ál. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34:2461-2498.
XIV. Klein TM, Blome C, Kleyn CE, Conrad C, et ál. Real-World Experience of Patient-Relevant Benefits and Treatment Satisfaction with Apremilast in Patients with Psoriasis: An Analysis of the APPRECIATE Study. Dermatol Ther. 2022;12:81-95.
XV. Galache‐Osuna C, Reyes‐García S, Salgueiro E, Bordallo‐Landa J, et ál. Retrospective study of apremilast drug survival in psoriasis patients in a daily practice setting: A long‐term experience. Dermatol Ther. 2022;35:e15583.
XVI. Carrascosa JM, Belinchón I, Rivera R, Ara M, et ál. The Use of Apremilast in Psoriasis: A Delphi Study. Actas Dermosifiliogr. 2020;111:115-134.
XVII. Amatore F, Villani AP, Tauber M, Viguier M, et ál. Psoriasis Research Group of the French Society of Dermatology (Groupe de Recherche sur le Psoriasis de la Société Française de Dermatologie). French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol. 2019;33:464-483.
XVIII. Keating GM. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs. 2017;77:459-472.
XIX. Gao JC, Wu AG, Contento MN, Maher JM, et ál. Apremilast in the Treatment of Plaque Psoriasis: Differential Use in Psoriasis. Clin Cosmet Investig Dermatol. 2022;15:395-402.
XX. Garbayo Salmons P, Expósito Serrano V, Romaní de Gabriel J, Ribera Pibernat M. Effectiveness and Drug Survival of Apremilast in 65 Patients With Psoriasis and/or Psoriatic Arthritis. Actas Dermosifiliogr. 2022;113:532-535.
XXI. Kaplan D, Husni E, Chang E, Broder MS, et ál. Biologic initiation rates in systemic-naive psoriasis patients after first-line apremilast versus methotrexate use. J Comp Eff Res. 2022;11:575-582.
Downloads
Published
Issue
Section
License
Copyright (c) 2022 on behalf of the authors. Reproduction rights: Argentine Society of Dermatology.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
El/los autor/es tranfieren todos los derechos de autor del manuscrito arriba mencionado a Dermatología Argentina en el caso de que el trabajo sea publicado. El/los autor/es declaran que el artículo es original, que no infringe ningún derecho de propiedad intelectual u otros derechos de terceros, que no se encuentra bajo consideración de otra revista y que no ha sido previamente publicado.
Le solicitamos haga click aquí para imprimir, firmar y enviar por correo postal la transferencia de los derechos de autor