Morpheas with cephalic manifestations
DOI:
https://doi.org/10.47196/da.v26i1.2060Keywords:
Linear morphea, Morphea en coup de sabre, Progressive facial hemiatrophy, Parry-Romberg SyndromeAbstract
Introduction: Progressive facial hemiatrophy (HFP) or Parry-Romberg Syndrome and Morphea en coup de sabre (MGS) are cephalic linear morpheas. They are chronics inflammatories diseases of the skin and underlying tissues, characterized by cutaneous atrophy and sclerosis.
Objectives: To describe clinical features, associated extracutaneous manifestations, histological and laboratory findings, imaging and diagnostic modalities and treatments established in patients with diagnosis of HFP, MGS, or both, evaluated in our Department.
Design: Retrospective descriptive study.
Materials and methods: We included medical histories of patients diagnosed with morphea evaluated in Collagenopathy Sector from July 2010 up to December 2016.
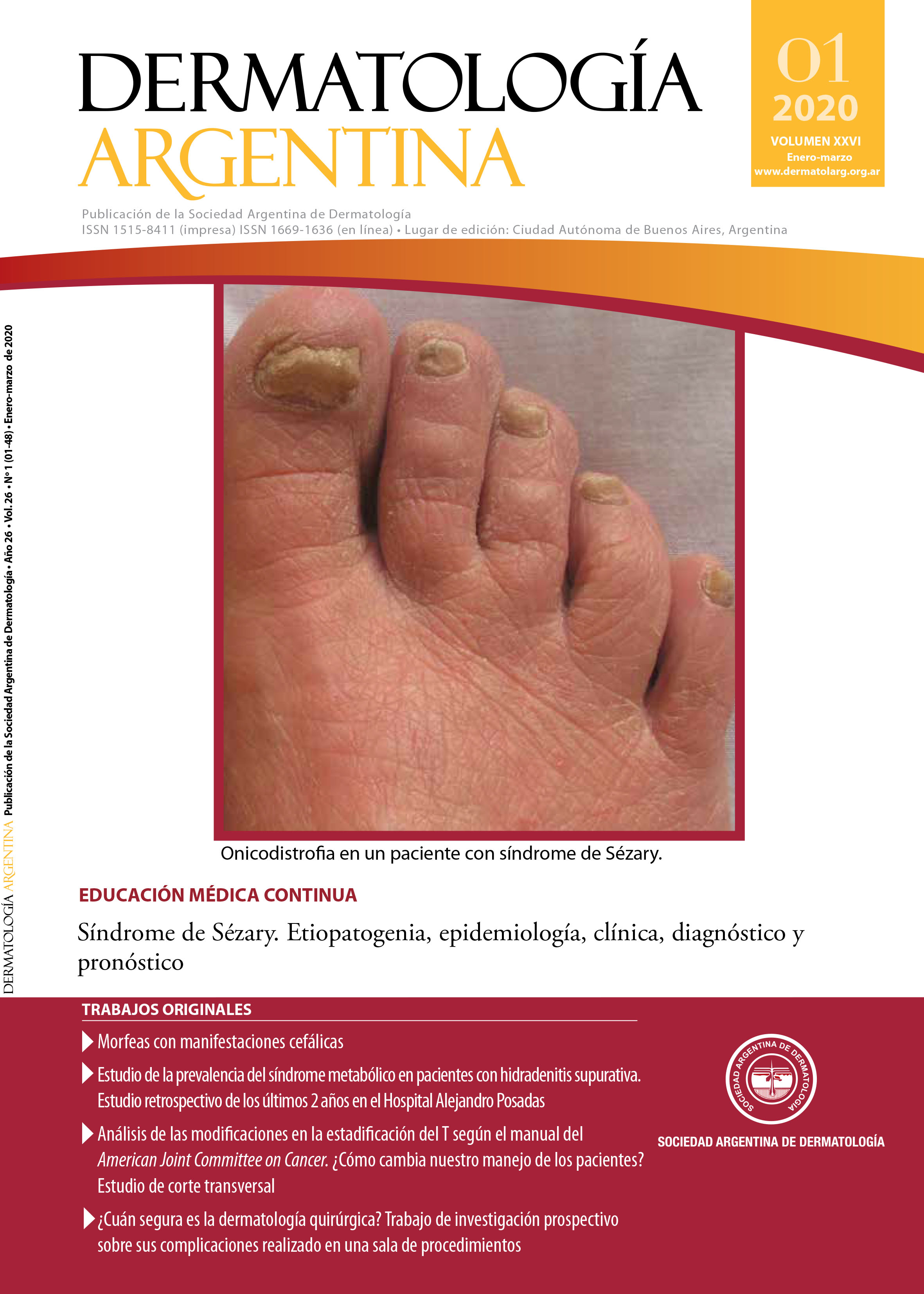
Results: Of 56 patients, 11 met the inclusion criteria, 7 with diagnosis of HFP, 2 with MGS and 2 with both pathologies. 64% were women. 64% showed extracutaneous manifestations. The treatment used in all of the patients was methotrexate, associated or not, with the use of systemic corticosteroids.
Conclusions: Most of our results agree with the bibliography consulted, with the exception of the associated manifestations. We emphasize the associated treatment of methotrexate and intravenous corticosteroid pulses with satisfactory results and well tolerated.
References
I. Figueiroa Careta M, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol 2015;90:62-73.
II. El-Kehdy J, Abbas O, Rubeiz N. A review of Parry-Romberg syndrome. J Am Acad Dermatol 2012;67:769-784.
III. Laxer LM, Zulian F. Localized scleroderma. Curr Opin Rheumatol 2006;18:606-613.
IV. Fett N, Werth V. Update on Morphea Part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2011;64: 217-228.
V. Zulian F, Athreya H, Laxer R, Nelson AM, et ál. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford) 2006;45:614-620.
VI. Bielsa Marsol I. Actualización en la clasificación y el tratamiento de la esclerodermia localizada. Actas Dermosifiliogr 2013;104:654-666.
VII. Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, et ál. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap síndromes. J Eur Acad Dermatol Venereol 2017;31:1401.1424.
VIII. Fett N, Werth V. Update on Morphea Part II. Outcome measures and treatment. J Am Acad Dermatol 2011;64:231-242.
IX. Kreuter A, Gambichler T, Breuckmann F, Rotterdam S, et ál. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol 2005;141:847-852.
X. Buján M, Merediz J, Nogales M, Cervini AB, et ál. Esclerodermia lineal en “coup de sabre” y síndrome de Parry-Romberg. Estudio retrospectivo en un hospital pediátrico. Arch Argent Dermatol 2009;59:43-52.
XI. Zulian F, Vallongo C, Patrizi A, Belloni-Fortina A, et ál. A long-term follow-up study of methotrexate in juvenile localized scleroderma (morphea). J Am Acad Dermatol 2012;67:1151-1156.
XII. Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol 2011;65:925-941.
XIII. Sommer A, Gambichler T, Bacharach-Buhles M, Von Rothenburg T, et ál. Clinical and serological characteristics of progressive facial hemiatrophy: a case series of 12 patients. J Am Acad Dermatol 2006;54:227-233.
Downloads
Published
Issue
Section
License
El/los autor/es tranfieren todos los derechos de autor del manuscrito arriba mencionado a Dermatología Argentina en el caso de que el trabajo sea publicado. El/los autor/es declaran que el artículo es original, que no infringe ningún derecho de propiedad intelectual u otros derechos de terceros, que no se encuentra bajo consideración de otra revista y que no ha sido previamente publicado.
Le solicitamos haga click aquí para imprimir, firmar y enviar por correo postal la transferencia de los derechos de autor