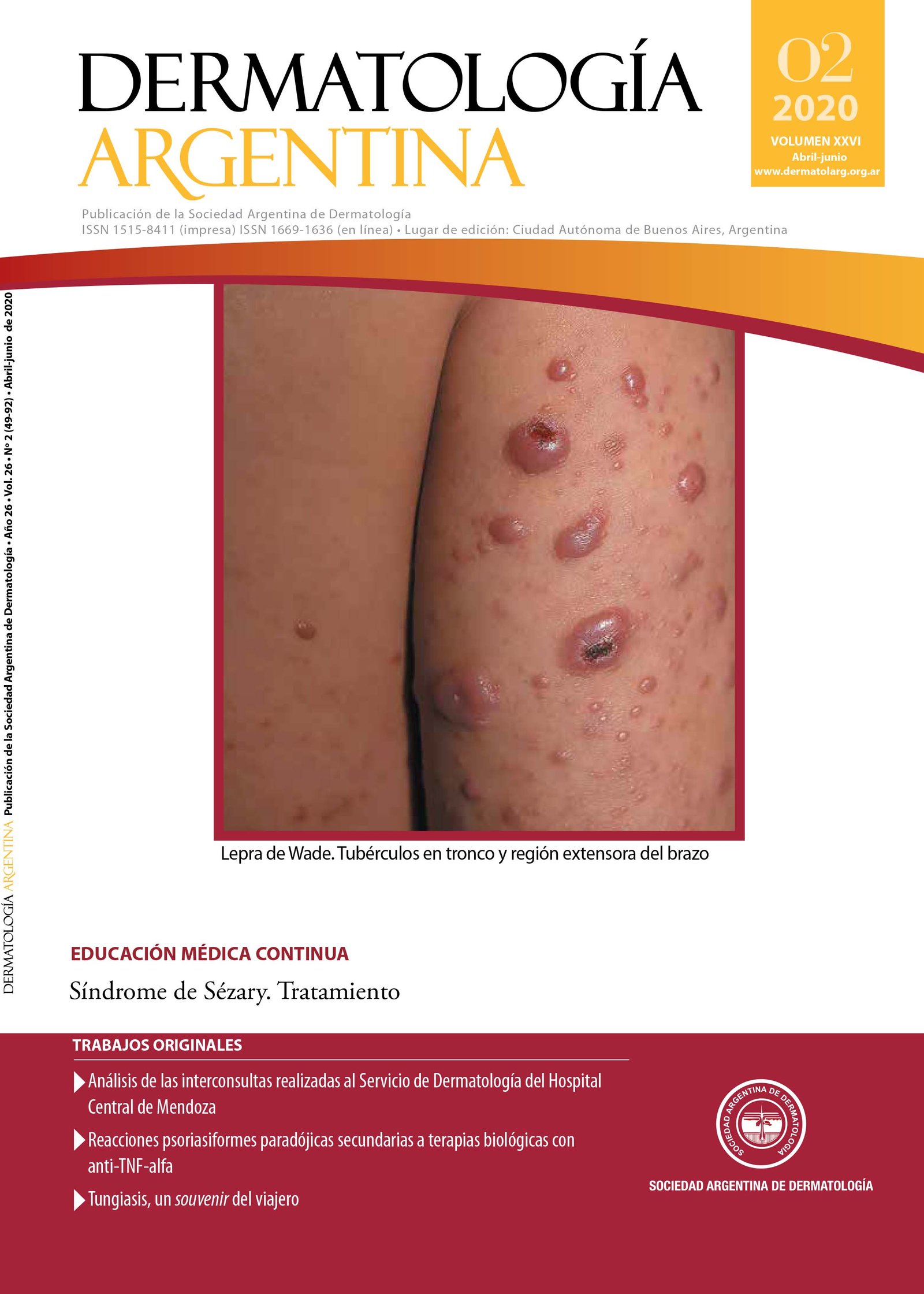
Síndrome de Sézary. Tratamiento
DOI:
https://doi.org/10.47196/da.v26i2.2074Palabras clave:
Síndrome de Sézary, Fototerapia, Fotoféresis extracorpórea, Retinoides, InterferónResumen
El síndrome de Sézary (SS) es una variante leucémica del linfoma cutáneo de células T con mal pronóstico, que requiere un abordaje multidisciplinario. El tratamiento es paliativo, ya que no se conocela curación para esta enfermedad. El objetivo terapéutico es reducir al mínimo las recurrencias y preservar la calidad de vida y la supervivencia global. Si bien se cuenta con numerosas opciones, las terapias inmunomoduladoras son las de elección. La quimioterapia se reserva para los casos refractarios o ante la progresión de la enfermedad.
Citas
I. Trautinger F, Eder J, Assaf C, Bagot M, et ál. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome – Update 2017. Eur JCancer 2017;77:57-74.
II. Mehta-Shah N, Horwitz SM, Ansell S, Ai WZ, et ál. Primary Cutaneous Lymphomas, Version 2.2020 Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw 2020;18:522-536.
III. Olsen EA, Rook AH, Zic J, Kim Y, et ál. Sézary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol 2011;64:352-404.
IV. Consenso Linfomas cutáneos primarios. Actualización 2018. Disponible en: <https://sad.org.ar/wp-content/uploads/2019/09/Consenso-Linfona-abril-2019.pdf>[Consultado mayo 2019].
V. Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, et ál. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): Part II. Prognosis, management, and future directions. J Am Acad Dermatol 2014;70:223e1-223e17.
VI. Trautinger F, Knobler R, Willemze R, Peris K, et ál. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer2006;42:1014-1030.
VII. Molgo M, Jaque A, Vial V, Ocqueteau M, et ál. Fotoféresis en el tratamiento de Síndrome de Sézary. Caso clínico. Rev Med Chile 2015;143:1449-1458.
VIII. Ram-Wolff C. Linfomas T cutáneos de tipo micosis fungoide/síndrome de Sézary (incluida parapsoriasis). EMC-Dermatología 2014;48:1-12.
IX. Willemze R, Hodak E, Zinzani PL, Specht L, et ál. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off 2013;24:149-154.
X. Horwitz SM, Olsen EA, Duvic M, Porcu P, et ál. Review of the treatment of mycosis fungoides and Sézary syndrome: a stage-based approach. J Natl Compr Canc Netw 2008;6:436-442.
XI. Olsen EA, Hodak E, Anderson T, Carter J B, et ál. Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium. J Am AcadDermatol 2016; 74: 27-58.
XII. Introcaso CE, Micaily B, Richardson SK, Junkins-Hopkins JM, et ál. Total skin electron beam therapy may be associated with improvement of peripheral blood disease in Sézary syndrome. J Am Acad Dermatol 2008;58:592-595.
XIII. Knobler R, Berlin G, Calzavara-Pinton P, Greinix H, et ál. Guidelines on the use of extracorporeal photopheresis. J Eur Acad Dermatol Venereol 2014;28:1-37.
XIV. Scarisbrick JJ, Taylor P, Holtick U, Makar Y, et ál. U.K. consensus statement on the use of extracorporeal photopheresis for treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Br J Dermatol 2008;158:659-678.
XV. Zic JA. Photopheresis in the treatment of cutaneous T-cell lymphoma: current status. Curr Opin Oncol 2012;24:1-10.
XVI. Olsen E. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther 2003;16:311-321.
XVII. Jumbou O, N´Guyen JM, Tessier MH, Legoux B, et ál. Long-term follow-up in 51 patients with micosis fungoides and Sézary síndrome treated by interferón-alfa. Br J Dermatol 1999;140:427-431.
XVIII. Papa G, Tura S, Mandelli F, Vegna ML, et ál. Is interferon alpha in cutaneous T-cell lymphoma a treatment of choice? Br J Dermatol 1991;79:48-51.
XIX. Kuzel TM, Gilyon K, Springer E, Variakojis D, et ál. Interferon alfa-2a combined with phototherapy in the treatment of cutaneous T-cell lymphoma. J Natl Cancer Inst 1990;82:203-207.
XX. Panchal M, Scarisbrick JJ. The utility of bexarotene in mycosisfungoides and Sézary syndrome. Onco TargetsTher 2015;8:367-373.
XXI. Duvic M, Hymes K, Heald P, Breneman D, et ál. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001;19:2456-2471.
XXII. Mann BS, Johnson JR, Cohen MH, Justice R, et ál. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007;12:1247-1252.
XXIII. Duvic M, Vu J. Update on the treatment of cutaneous T-cell lymphoma (LCCT): focus on vorinostat. Biologics2007;1:377-392.
XXIV. Shah RR. Safety and Tolerability of Histone Deacetylase (HDAC) Inhibitors in Oncology. Drug Saf 2019;42:235-245.
XXV. Kim YH, Tavallaee M, Sundram U, Salva KA, et ál. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: a multi-institution collaborative project. J Clin Oncol 2015;33:3750-3758.
XXVI. Prince HM, Kim YH, Horwitz SM, Dummer R, et ál. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 2017;390:555-566.
XXVII. Hughes C, Khot A, Mc Cormack C, Lade S, et ál. Lack of durable disease control with chemotherapy for mycosis fungoides and Sézary syndrome: a comparative study of systemic therapy. Blood 2015;125:71-81.
XXVIII. Zackheim HS, Epstein EH Jr. Low-dose methotrexate for the Sezary syndrome. J Am Acad Dermatol 1989;21:757-762.
XXIX. Molina A, Zain J, Arber DA, Angelopolou M, et ál. Durable Clinical, Cytogenetic, and Molecular Remissions After Allogeneic Hematopoietic Cell Transplantation for Refractory Sezary Syndrome and Mycosis Fungoides. J Clin Oncol 2005;23:6163-6171.
XXX. Meyer N, Paul C, Misery L. Pruritus in Cutaneous T-cell Lymphomas: Frequent, Often Severe and Difficult to Treat. Acta Derm Venereol 2010;90:12-17.
XXXI. Görge T, Schiller M. Pruritus in Cutaneous T-cell Lymphoma, en Misery L, Stander S. Pruritus. Londres: Springer;2010:121-124.
XXXII. Brune A, Metze D, Luger TA, Ständer S. Antipruritic therapy with the oral opioid receptor antagonist naltrexone. Open, non placebo controlled administration in 133 patients. Hautarzt 2004;55:1130-1136.
Descargas
Publicado
Número
Sección
Licencia
El/los autor/es tranfieren todos los derechos de autor del manuscrito arriba mencionado a Dermatología Argentina en el caso de que el trabajo sea publicado. El/los autor/es declaran que el artículo es original, que no infringe ningún derecho de propiedad intelectual u otros derechos de terceros, que no se encuentra bajo consideración de otra revista y que no ha sido previamente publicado.
Le solicitamos haga click aquí para imprimir, firmar y enviar por correo postal la transferencia de los derechos de autor